SOP - ABR --PUBLIC: Difference between revisions
No edit summary |
Wardchriss (talk | contribs) No edit summary |
||
| (3 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
'''Auditory Brainstem Response (ABR)''' | '''Auditory Brainstem Response (ABR)''' | ||
= Purpose = | |||
ABR testing is used to assess an animals ability to hear sound, either as multifrequency clicks or at defined frequencies | |||
= Experimental Design = | |||
'''Minimum number of animals:''' Recommend at least 5 animals per group being compared - though IMPC requirement is 2 per sex per group | |||
'''Age at test: ''' Recommended >3wks, commonly performed on mice >12wks | |||
''' | '''Sex:''' either | ||
'''Time of Day:''' typically during the light phase, but may vary depending on study requirements | |||
= Equipment = | |||
* Sound and EM shielded box | |||
* Specialty microphone and speaker | |||
* Warming Pad (One for the Testing Chamber, and larger cage size pads for the cages as animals wait for the test, or recover from anesthesia | |||
* Amplifier and Signal Conditioning System (TDT Medusa) | |||
* Oscilloscope | |||
Consumable or Supplies Needed | |||
* Needle electrodes (3 channel) | |||
* Eye ointment | |||
* 1cc syringes | |||
* Anesthesia Cocktail - Ketamine/Xylazine (10mg/kg, 1mg/kg) | |||
= Procedure = | |||
== Protocol == | |||
=== Equipment setup === | |||
* Turn on PC monitor. [Do not log out at any time before or following the procedure.] | |||
* All ABR equipment should be switched on a minimum of 5 minutes before beginning the experiment in order to reach optimal operation conditions. This includes: | |||
** Turning on the oscilloscope | |||
** Switching on the heating pad | |||
** Switching on all four amplifier and signal conditioning boxes | |||
** Switching on the microphone amplifier (and confirming battery has sufficient power) | |||
** Turning on the needle electrode amplifier and unplugging the charging cord | |||
* Arrange cages to be tested in the order in which they appear on the BaSH experimental worksheet. | |||
=== Mouse Weighing and Logs === | |||
For IMPC/KOMP testing, mice are tested in an order predetermined by the BaSH sheet, sent weekly. Logs of each animal’s statistics are to be hand-written in the ABR notebook, then transferred to the PC. | |||
For local/PI projects, a log sheet for each animals biometric statistics is to be hand-written, and transcribed to the project folder which will be shared with the PI. | |||
* After turning on all ABR equipment, select the first mouse to be tested and weigh on the mouse scale. Record the mouse’s weight in the notebook. Replace mouse and move on to the next if applicable for the cage. | |||
* The mouse’s ID and genotype, the amount of anaesthetic drug to be used and the time of injection, are to be recorded in the ABR notebook. | |||
==== Notebook Informatics ==== | |||
The ABR notebook contains the following information for each mouse to be tested | The ABR notebook contains the following information for each mouse to be tested | ||
* Mouse name (ID) | |||
* Mouse strain (genotype) | |||
* Dosage given (If mouse weighs 20.0g instead of giving .200ml dose subtract .03ml to get .170ml. Do this for all mice) | |||
* Weight of mouse | |||
* ABR range of decibels (0-85 or 0-95) | |||
=== Mouse Anesthesia === | |||
* When all equipment is operational, select the mouse to be tested. | * When all equipment is operational, select the mouse to be tested. | ||
| Line 52: | Line 68: | ||
* Place the injected mouse back into its home cage. | * Place the injected mouse back into its home cage. | ||
=== Calibrate sound system and software === | |||
Before the first mouse of the day is tested, the ABR system should be calibrated. A calibration file will be generated and used in the testing of all mice that day. | Before the first mouse of the day is tested, the ABR system should be calibrated. A calibration file will be generated and used in the testing of all mice that day. | ||
==== For 1<sup>st</sup> mouse tested ==== | |||
* Double-click on the “Averager” program to open it | * Double-click on the “Averager” program to open it | ||
| Line 71: | Line 83: | ||
* Ensure “Save Calibration to File” is checked. | * Ensure “Save Calibration to File” is checked. | ||
* Click “Run”. | * Click “Run”. | ||
* When recording is complete, enter calibration filename as the date DDMMYY) and press “OK”. | * When recording is complete, enter calibration filename as the date (DDMMYY) and press “OK”. | ||
=== Mouse Preparation for Recording === | |||
* Confirm mouse is fully anesthetized by pinching the toe and checking for a reflex. | * Confirm mouse is fully anesthetized by pinching the toe and checking for a reflex. | ||
* Remove the microphone and set the mouse on the heating blanket within the sound chamber in its place. Mouse should be at a distance of 10-20 centimeters from the loudspeaker. | * Remove the microphone and set the mouse on the heating blanket within the sound chamber in its place. Mouse should be at a distance of 10-20 centimeters from the loudspeaker. | ||
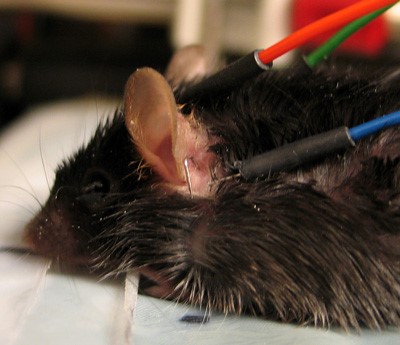
* Insert needle electrodes (active electrode on the vertex, reference electrode overlying the left bulla, ground electrode overlying the right bulla). See Fig. 1 | * Insert needle electrodes (active electrode on the vertex, reference electrode overlying the left bulla, ground electrode overlying the right bulla). See Fig. 1 | ||
* There is a sign in the | * There is a sign in the chamber that says which side of the mouse each colored electrode corresponds to. | ||
* Note the ECG trace on the oscilloscope to ensure that electrodes have been inserted correctly. The reading should yield a low-noise trace with a 2-5V peak. | * Note the ECG trace on the oscilloscope to ensure that electrodes have been inserted correctly. The reading should yield a low-noise trace with a 2-5V peak. | ||
* Close the chamber door and lock into place. | * Close the chamber door and lock into place.<br /> | ||
=== Auditory Brainstem Response Recording === | |||
==== Record “Click ABR” – to confirm recording apparatus is functioning ==== | |||
* Click on: '''''File>Load Setup>Auditory Brainstem Response>Click ABR.''''' | * Click on: '''''File>Load Setup>Auditory Brainstem Response>Click ABR.''''' | ||
| Line 94: | Line 104: | ||
* If necessary, make adjustments to electrode position. | * If necessary, make adjustments to electrode position. | ||
==== Record an ECG trace to determine mouse heartrate ==== | |||
* Click on: '''''File>Load Setup>Heart Rate>Estimate Heart Rate.''''' | * Click on: '''''File>Load Setup>Heart Rate>Estimate Heart Rate.''''' | ||
* Hit “Run” to record a single sweep, 5s in duration, to display the ECG. | * Hit “Run” to record a single sweep, 5s in duration, to display the ECG. | ||
==== Record Click-Evoked ABRs for determination of click threshold ==== | |||
* Click on: '''''File>Load Setup>ABR MGP Screen>Click.''''' | * Click on: '''''File>Load Setup>ABR MGP Screen>Click.''''' | ||
* Hit Run. | * Hit Run. | ||
ABR recordings will be made using an array of click stimuli (10µs duration, 42.6/sec, 256 sweeps), from 0 - 85dB SPL in 5dB steps. | ABR recordings will be made using an array of click stimuli (10µs duration, 42.6/sec, 256 sweeps), from 0 - 85dB SPL in 5dB steps. | ||
The data collected will be displayed in real time on the right-hand monitor, to aid immediate visual interpretation of results. | The data collected will be displayed in real time on the right-hand monitor, to aid immediate visual interpretation of results. | ||
==== Record Tone-evoked ABRs for determination of tone thresholds ==== | |||
* Click on: '''''File>Load Setup>ABR MGP Screen>30-6 kHz.''''' | * Click on: '''''File>Load Setup>ABR MGP Screen>30-6 kHz.''''' | ||
** the 30-6 kHz setup goes up to 85db, you can manually raise the upper limit (recommended to increase to 95db for 18, 24, and 30 kHz) | |||
** additional profiles that include 3 kHz or 42 KHz can be selected and run if specified by the project (not applicable to IMPC/KOMP) | |||
* Hit Run. | * Hit Run. | ||
ABR recordings will be made using an array of stimuli (frequencies and levels). The run will present 5ms long tone-pips (1ms rise/fall time, 42.6/sec, 256 sweeps) across 5 frequencies (30, 24, 18, 12, 6 kHz) at intensity levels from 0 - 85dB SPL in 5dB steps. Like the click data, the sweep data collected will be displayed in real time on the right-hand monitor. | ABR recordings will be made using an array of stimuli (frequencies and levels). The run will present 5ms long tone-pips (1ms rise/fall time, 42.6/sec, 256 sweeps) across 5 frequencies (30, 24, 18, 12, 6 kHz) at intensity levels from 0 - 85dB SPL in 5dB steps. Like the click data, the sweep data collected will be displayed in real time on the right-hand monitor. | ||
'''Note:''' many mice do not show visible ABR thresholds at the 24kHz and 30kHz frequency levels. Should a test mouse fall into this category, extra stimuli must be presented up to 95dB. After the 0-85dB sweep is completed across each frequency level, check the box reading “up to 95dB” and input the start level at 90dB. Then hit Run to collect the extra data. Note the 95dB max range in the ABR notebook. | '''Note:''' many mice do not show visible ABR thresholds at the 24kHz and 30kHz frequency levels. Should a test mouse fall into this category, extra stimuli must be presented up to 95dB. After the 0-85dB sweep is completed across each frequency level, check the box reading “up to 95dB” and input the start level at 90dB. Then hit Run to collect the extra data. Note the 95dB max range in the ABR notebook. | ||
The “Move On” button can be used to speed up recording – use only when confident there is a clear ABR waveform present. The “Pause” button can used to temporarily halt recording should the need arise (after the current stimulus has finished). Recording may be paused in order to check on the mouse, re-adjust the needle electrodes, et cetera. | The “Move On” button can be used to speed up recording – use only when confident there is a clear ABR waveform present. The “Pause” button can used to temporarily halt recording should the need arise (after the current stimulus has finished). Recording may be paused in order to check on the mouse, re-adjust the needle electrodes, et cetera. | ||
==== Prepare Click-evoked ABR data for export ==== | |||
While the computer collects the tone-evoked ABR dataset (section 1.6.4), use | While the computer collects the tone-evoked ABR dataset (section 1.6.4), use “Traceview” to export click-evoked ABR data for peak analysis. | ||
* Run “Traceview”. | * Run “Traceview”. | ||
| Line 140: | Line 142: | ||
* Exit “Traceview”. | * Exit “Traceview”. | ||
==== Prepare Click-evoked ABR data for Phase 3 (IOF) Analysis ==== | |||
While the computer collects the tone-evoked ABR dataset (section 1.5.4), use “ABR Notepad” to generate and export the data required for Input-Output Function (Phase 3) analysis. | While the computer collects the tone-evoked ABR dataset (section 1.5.4), use “ABR Notepad” to generate and export the data required for Input-Output Function (Phase 3) analysis. | ||
* Run | * Run “ABR Notepad”. | ||
* Drag | * Drag and drop the file (“mouseID.csv_click_for ABR notebook.txt”) into the ABR Notepad window. | ||
* Position | * Position markers (1 & 2) onto positive and negative peaks of wave 1 and wave 3. Markers 3, 4, and 5 are moved to the far right of the configuration and left there. | ||
* Hit | * Hit “s” to save the amplitude and latency information for later use in Phase 3 analysis (if required). | ||
* A | * A new txt file is saved (“mouseID.csv”_click_for ABR notebook.txt-analysed.txt”). | ||
Once collection of the tone-evoked ABR dataset (section 1.6.4) is complete, proceed to: | Once collection of the tone-evoked ABR dataset (section 1.6.4) is complete, proceed to: | ||
==== Record an ECG trace to determine heartrate (once section 1.6.4 is done) ==== | |||
* Click on : '''''File>Load | * Click on: '''''File>Load Setup>Heart Rate>Estimate Heart Rate''''' | ||
* Hit “Run” to record a | * Hit “Run” to record a single sweep, 5s in duration, to display a second ECG trace. | ||
This concludes the ABR screen recording protocol. The mouse should now be removed from the sound chamber and passed to the next experimenter to be X-rayed. | |||
== ABR data Analysis == | |||
Data files for each tested mouse are to be analyzed, processed in Excel, and uploaded to the database. | |||
=== Analysis of ABR Data === | |||
· Run “Traceview 2.0”. | · Run “Traceview 2.0”. | ||
· Click on: File>Load, and select the ABR data file for the | · Click on: File>Load, and select the ABR data file for the previous mouse. | ||
· In the tracelist, select the click ABRs recorded from the 0-85dB SPL series (use “select block” here). | · In the tracelist, select the click ABRs recorded from the 0-85dB SPL series (use “select block” here). | ||
| Line 190: | Line 188: | ||
· Open the previously uploaded data in Excel by selecting '''mpc(\\10.20.202.104) (Y:)>BaSH | === Preparring Excel data for LIMS Upload (for IMPC/KOMP testing) === | ||
· Open the previously uploaded data in Excel by selecting '''mpc(\\10.20.202.104) (Y:)>ABR>Uploaded BaSH Spreadsheets''' | |||
· Using the top bar information as a template, open a new Excel file for the data to be uploaded | · Using the top bar information as a template, open a new Excel file for the data to be uploaded | ||
| Line 204: | Line 203: | ||
· Paste the transposed data into your aggregate data Excel sheet. Close the other file and move on to the next mouse. | · Paste the transposed data into your aggregate data Excel sheet. Close the other file and move on to the next mouse. | ||
· When data compilation is complete, save as | · When data compilation is complete, save as “uploadedDDMMYY” using that day’s date. File should be saved as a .csv (comma delimited) extension. | ||
=== Uploading Aggregated ABR Data to LIMS (IMPC/KOMP)=== | |||
· Visit the LIMS homepage | |||
· Click: Phenotyping>Data Capture>File Upload and fill out the requested fields. | · Click: Phenotyping>Data Capture>File Upload and fill out the requested fields. | ||
| Line 224: | Line 221: | ||
· Click “Save” to upload data. | · Click “Save” to upload data. | ||
==== Processing Uploaded ABR Data ==== | |||
· On the database, click: Phenotyping>Data Capture>List Phenotyping Work | · On the database, click: Phenotyping>Data Capture>List Phenotyping Work | ||
| Line 233: | Line 229: | ||
· Under Filtering, select: “DCF>is equal to>Auditory Brainstem Response” followed by “Completed>is equal to>No | · Under Filtering, select: “DCF>is equal to>Auditory Brainstem Response” followed by “Completed>is equal to>No | ||
· Under Sorting, select: | · Under Sorting, select: “Procedure Date>ascending>blanks last” | ||
· Click “show records” to display uncompleted data | · Click “show records” to display uncompleted data | ||
| Line 259: | Line 255: | ||
|b. Reference electrode in position behind the left ear. | |b. Reference electrode in position behind the left ear. | ||
|} | |} | ||
* | |||
= Data Outcome Measures Produced = | = Data Outcome Measures Produced = | ||
| Line 265: | Line 261: | ||
=== Common parameters of interest === | === Common parameters of interest === | ||
* ''' | * '''db Threshold for Click''' : db threshold for an observable ABR waveform to be detected using clicks | ||
* ''' | * '''db Threshold for frequency (3kHz - 42kHz)''' : db threshold for an observable ABR waveform to be detected using specific frequencies | ||
[[category: Shareable SOPs --PUBLIC]] | [[category: Shareable SOPs --PUBLIC]] | ||
[[category: Standard Operating Procedures/Protocols]] | [[category: Standard Operating Procedures/Protocols]] | ||
Latest revision as of 23:10, 9 February 2024
Auditory Brainstem Response (ABR)
Purpose
ABR testing is used to assess an animals ability to hear sound, either as multifrequency clicks or at defined frequencies
Experimental Design
Minimum number of animals: Recommend at least 5 animals per group being compared - though IMPC requirement is 2 per sex per group
Age at test: Recommended >3wks, commonly performed on mice >12wks
Sex: either
Time of Day: typically during the light phase, but may vary depending on study requirements
Equipment
- Sound and EM shielded box
- Specialty microphone and speaker
- Warming Pad (One for the Testing Chamber, and larger cage size pads for the cages as animals wait for the test, or recover from anesthesia
- Amplifier and Signal Conditioning System (TDT Medusa)
- Oscilloscope
Consumable or Supplies Needed
- Needle electrodes (3 channel)
- Eye ointment
- 1cc syringes
- Anesthesia Cocktail - Ketamine/Xylazine (10mg/kg, 1mg/kg)
Procedure
Protocol
Equipment setup
- Turn on PC monitor. [Do not log out at any time before or following the procedure.]
- All ABR equipment should be switched on a minimum of 5 minutes before beginning the experiment in order to reach optimal operation conditions. This includes:
- Turning on the oscilloscope
- Switching on the heating pad
- Switching on all four amplifier and signal conditioning boxes
- Switching on the microphone amplifier (and confirming battery has sufficient power)
- Turning on the needle electrode amplifier and unplugging the charging cord
- Arrange cages to be tested in the order in which they appear on the BaSH experimental worksheet.
Mouse Weighing and Logs
For IMPC/KOMP testing, mice are tested in an order predetermined by the BaSH sheet, sent weekly. Logs of each animal’s statistics are to be hand-written in the ABR notebook, then transferred to the PC.
For local/PI projects, a log sheet for each animals biometric statistics is to be hand-written, and transcribed to the project folder which will be shared with the PI.
- After turning on all ABR equipment, select the first mouse to be tested and weigh on the mouse scale. Record the mouse’s weight in the notebook. Replace mouse and move on to the next if applicable for the cage.
- The mouse’s ID and genotype, the amount of anaesthetic drug to be used and the time of injection, are to be recorded in the ABR notebook.
Notebook Informatics
The ABR notebook contains the following information for each mouse to be tested
- Mouse name (ID)
- Mouse strain (genotype)
- Dosage given (If mouse weighs 20.0g instead of giving .200ml dose subtract .03ml to get .170ml. Do this for all mice)
- Weight of mouse
- ABR range of decibels (0-85 or 0-95)
Mouse Anesthesia
- When all equipment is operational, select the mouse to be tested.
- Remove mouse from its cage and note ear-mark to verify its identity.
- Intraperitoneally inject mouse with the proper dose of prepared ketamine/xylazine (0.1ml /10g; a 20 gram mouse would receive a 0.170 milliliter dosage).
- Place the injected mouse back into its home cage.
Calibrate sound system and software
Before the first mouse of the day is tested, the ABR system should be calibrated. A calibration file will be generated and used in the testing of all mice that day.
For 1st mouse tested
- Double-click on the “Averager” program to open it
- On the “Animal and Experiment Information” window, complete the requested fields. Information to be input includes mouse ID, weight, and date of birth, and dose/drug given.
- Ensure no filename is selected in the calibration list-box.
- Click “Proceed”.
- Place the microphone in position in the sound booth and close door.
- Click on: File>Load Setup>Calibration>Calibration by White Noise.
- Ensure “Save Calibration to File” is checked.
- Click “Run”.
- When recording is complete, enter calibration filename as the date (DDMMYY) and press “OK”.
Mouse Preparation for Recording
- Confirm mouse is fully anesthetized by pinching the toe and checking for a reflex.
- Remove the microphone and set the mouse on the heating blanket within the sound chamber in its place. Mouse should be at a distance of 10-20 centimeters from the loudspeaker.
- Insert needle electrodes (active electrode on the vertex, reference electrode overlying the left bulla, ground electrode overlying the right bulla). See Fig. 1
- There is a sign in the chamber that says which side of the mouse each colored electrode corresponds to.
- Note the ECG trace on the oscilloscope to ensure that electrodes have been inserted correctly. The reading should yield a low-noise trace with a 2-5V peak.
- Close the chamber door and lock into place.
Auditory Brainstem Response Recording
Record “Click ABR” – to confirm recording apparatus is functioning
- Click on: File>Load Setup>Auditory Brainstem Response>Click ABR.
- Hit “Run” to present a test click stimuli (256 clicks @ 70dB SPL) to ensure a good ABR is evoked. Click ABR should be free of noise/sinusoidal wave interference.
- If necessary, make adjustments to electrode position.
Record an ECG trace to determine mouse heartrate
- Click on: File>Load Setup>Heart Rate>Estimate Heart Rate.
- Hit “Run” to record a single sweep, 5s in duration, to display the ECG.
Record Click-Evoked ABRs for determination of click threshold
- Click on: File>Load Setup>ABR MGP Screen>Click.
- Hit Run.
ABR recordings will be made using an array of click stimuli (10µs duration, 42.6/sec, 256 sweeps), from 0 - 85dB SPL in 5dB steps.
The data collected will be displayed in real time on the right-hand monitor, to aid immediate visual interpretation of results.
Record Tone-evoked ABRs for determination of tone thresholds
- Click on: File>Load Setup>ABR MGP Screen>30-6 kHz.
- the 30-6 kHz setup goes up to 85db, you can manually raise the upper limit (recommended to increase to 95db for 18, 24, and 30 kHz)
- additional profiles that include 3 kHz or 42 KHz can be selected and run if specified by the project (not applicable to IMPC/KOMP)
- Hit Run.
ABR recordings will be made using an array of stimuli (frequencies and levels). The run will present 5ms long tone-pips (1ms rise/fall time, 42.6/sec, 256 sweeps) across 5 frequencies (30, 24, 18, 12, 6 kHz) at intensity levels from 0 - 85dB SPL in 5dB steps. Like the click data, the sweep data collected will be displayed in real time on the right-hand monitor.
Note: many mice do not show visible ABR thresholds at the 24kHz and 30kHz frequency levels. Should a test mouse fall into this category, extra stimuli must be presented up to 95dB. After the 0-85dB sweep is completed across each frequency level, check the box reading “up to 95dB” and input the start level at 90dB. Then hit Run to collect the extra data. Note the 95dB max range in the ABR notebook.
The “Move On” button can be used to speed up recording – use only when confident there is a clear ABR waveform present. The “Pause” button can used to temporarily halt recording should the need arise (after the current stimulus has finished). Recording may be paused in order to check on the mouse, re-adjust the needle electrodes, et cetera.
Prepare Click-evoked ABR data for export
While the computer collects the tone-evoked ABR dataset (section 1.6.4), use “Traceview” to export click-evoked ABR data for peak analysis.
- Run “Traceview”.
- Click on: File>Load, and select the ABR data file for the current mouse.
- In the tracelist, select the click ABRs recorded from the 0-85dB SPL series (use “select block” here).
- Click on: Plot>Export selected traces for peak analysis, to generate the file needed for input/output function analysis.
- Exit “Traceview”.
Prepare Click-evoked ABR data for Phase 3 (IOF) Analysis
While the computer collects the tone-evoked ABR dataset (section 1.5.4), use “ABR Notepad” to generate and export the data required for Input-Output Function (Phase 3) analysis.
- Run “ABR Notepad”.
- Drag and drop the file (“mouseID.csv_click_for ABR notebook.txt”) into the ABR Notepad window.
- Position markers (1 & 2) onto positive and negative peaks of wave 1 and wave 3. Markers 3, 4, and 5 are moved to the far right of the configuration and left there.
- Hit “s” to save the amplitude and latency information for later use in Phase 3 analysis (if required).
- A new txt file is saved (“mouseID.csv”_click_for ABR notebook.txt-analysed.txt”).
Once collection of the tone-evoked ABR dataset (section 1.6.4) is complete, proceed to:
Record an ECG trace to determine heartrate (once section 1.6.4 is done)
- Click on: File>Load Setup>Heart Rate>Estimate Heart Rate
- Hit “Run” to record a single sweep, 5s in duration, to display a second ECG trace.
This concludes the ABR screen recording protocol. The mouse should now be removed from the sound chamber and passed to the next experimenter to be X-rayed.
ABR data Analysis
Data files for each tested mouse are to be analyzed, processed in Excel, and uploaded to the database.
Analysis of ABR Data
· Run “Traceview 2.0”.
· Click on: File>Load, and select the ABR data file for the previous mouse.
· In the tracelist, select the click ABRs recorded from the 0-85dB SPL series (use “select block” here).
· Hit “CTRL + s” and locate the threshold for the click data on the screen.
· For each frequency level (6k-30k Hz), hit “CTRL + f” followed by “CTRL + s” to plot waveforms and locate the decibel threshold of hearing for that frequency.
· In Excel, locate and open the file for the current mouse’s genotype. If no spreadsheet exists for that gene knockout, create a new one using the template from a pre-existing document of the same type.
· Record the click and tone thresholds of each mouse into excel according to the template.
Preparring Excel data for LIMS Upload (for IMPC/KOMP testing)
· Open the previously uploaded data in Excel by selecting mpc(\\10.20.202.104) (Y:)>ABR>Uploaded BaSH Spreadsheets
· Using the top bar information as a template, open a new Excel file for the data to be uploaded
· The format for experimental date/time should be customized as follows in Excel: “dd-mmm-yyyy hh:mm:ss AM/PM”. The data must be in this format to be uploaded to LIMS.
· To input each mouse’s data, open its genotype Excel sheet (contained under mpc(\\10.20.202.104) (Y:)>ABR>ABR Analyzed). Select the mouse ID, then hit CTRL and also select the click and frequency thresholds. Include the blank space above the click data threshold.
· Select a random blank square. Right click and select “transpose” to transpose the data. Copy.
· Paste the transposed data into your aggregate data Excel sheet. Close the other file and move on to the next mouse.
· When data compilation is complete, save as “uploadedDDMMYY” using that day’s date. File should be saved as a .csv (comma delimited) extension.
Uploading Aggregated ABR Data to LIMS (IMPC/KOMP)
· Visit the LIMS homepage
· Click: Phenotyping>Data Capture>File Upload and fill out the requested fields.
o SOP: Auditory Brainstem Response.
o Upload File: the newly saved aggregate file.
o First Row of Data and Field Separator are left at “1” and “COMMA” respectively.
o Technician: click “My PIL” to enter your credentials.
· Click “Save” to upload data.
Processing Uploaded ABR Data
· On the database, click: Phenotyping>Data Capture>List Phenotyping Work
· Hit “recall” to display previous fields
· Under Filtering, select: “DCF>is equal to>Auditory Brainstem Response” followed by “Completed>is equal to>No
· Under Sorting, select: “Procedure Date>ascending>blanks last”
· Click “show records” to display uncompleted data
· Select an uncompleted DCF and click “edit”. For all mice which read “Edit” under the Options section, open the file and scroll down to the bottom right corner of the form. Click the open box to complete that DCF. Data should be automatically present upon opening the form.
· For all mice which read “Start” under the Options section, click “Start” and change the experimental date to match the scheduled date. On the BaSH sheet, verify that the mouse was not an ABR test mouse. Find the droplist at the bottom of the screen and select “IMPC_PSC_015: Not tested due to Sufficient mice to meet IMPC Requirement”. Click the complete box to complete the form.
· When all DCFs have been completed (check that each has a green check mark), click “Complete All” and check the “Work Finished” box at the top of the screen. Then click “Save”.
· Continue until all data has been processed.
This concludes the ABR analysis and uploading protocol.
Fig. 1 & 2. To indicate positioning of sub-dermal needle electrodes for ABR recording.
| a. Active electrode in position behind on the vertex. |
| b. Reference electrode in position behind the left ear. |
Data Outcome Measures Produced
Common parameters of interest
- db Threshold for Click : db threshold for an observable ABR waveform to be detected using clicks
- db Threshold for frequency (3kHz - 42kHz) : db threshold for an observable ABR waveform to be detected using specific frequencies